HDRP Newsletter, February 2021
Message from the HDRP Associate Director

On behalf of all my colleagues in the Healthcare Delivery Research Program (HDRP), I wish you a happy and a healthy new year.
Although 2020 has given way to 2021 and a new administration, many of the same challenges still confront us as individuals and as a society. With all that’s going on, I continue to be humbled by the dedication and creativity of the research community in responding to these challenges. We in HDRP also remain dedicated to our mission of continuing to work with you to advance our shared goal of advancing innovative research to improve the delivery of cancer-related care.
In this issue of the newsletter, we provide a summary of HDRP’s FY2020 Grants Portfolio, as well as information about funding opportunities, upcoming events, and data resources supported by HDRP. We are excited to announce that you can now view a sampling of HDRP’s funded applications via our website -- we hope you will find this useful. Additionally, this newsletter includes links to COVID-19 initiatives at NIH and NCI and funding opportunities for COVID-19 research.
HDRP is also excited to share information about the highly successful 2020 Multilevel Intervention Training Institute and the Future of Cancer Health Economics Research Virtual Conference. Please also take moment to read about our 2019-2020 NCI/AcademyHealth Visiting Scholar, Dr. Shellie Ellis’ research efforts and note that we are currently recruiting for the 2021-2022 Visiting Scholar(s). Applications are due by March 22.
I encourage you to reach out to me or any of our staff to learn more about these activities and funding opportunities. A full staff listing is available on our website.
Announcements
HDRP FY2020 Grant Portfolio
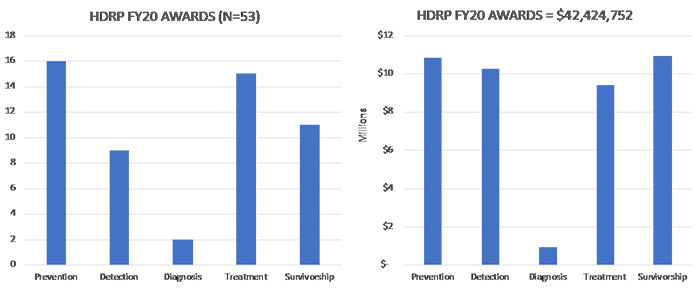
In FY 2020, HDRP funded 53 new grants, totaling over $42 million dollars. These grants supported research across the cancer continuum to improve cancer prevention, detection, diagnosis, treatment and survivorship care.
For a complete listing of all grants currently funded by HDRP, click here .
.
Introducing HDRP Sample Grants!
We are happy to announce that you can now view a sampling of HDRP’s funded applications via our website.
NCI frequently receives requests for examples of funded grant applications. Several investigators and their organizations agreed to let HDRP post excerpts of their healthcare delivery research grant applications online.
We currently have 7 funded grant applications posted as a resource for potential applicants to see examples of excellent, funded proposal in healthcare delivery research.
We are grateful to the investigators and their institutions for allowing us to provide this important resource to the community.
Individuals using assistive technology (e.g., screen reader, Braille reader) who experience difficulty accessing any information should send an email to the Healthcare Delivery Research Program.
Now accepting applications for the 2021-2022 NCI/AcademyHealth Visiting Scholars Program!
Are you interested in learning more about the NCI/AcademyHealth Visiting Scholars Program? Applications for the next Scholar are now being accepted through March 22, 2021. Click here to learn more and apply. To read more about this year’s Scholar, Dr. Shellie Ellis’ work, look in the Research Highlights section of this Newsletter.
Policy Updates
The final NCI funding policies for FY2021 are available here . Additionally, paylines for several frequently used funding mechanisms are:
. Additionally, paylines for several frequently used funding mechanisms are:
| Mechanism | Funding Range |
|---|---|
| Experienced and New Investigator R01 | 11th percentile |
| Early-stage Investigator R01 | 16th percentile |
| Exploratory / Dev (R21) | 9th percentile |
| Small Grant (R03) | 25 Impact Score |
NIH Expanding Usage of Notices of Special Interest (NOT-OD-19-107)
This Notice informs the extramural community that NIH is expanding and formalizing the use of Notices of Special Interest (NOSI) posted in the NIH Guide for Grants and Contracts to announce interest in specific scientific research topics.
How Does a NOSI Work?
A NOSI is a standard, formal format for NIH institutes to share and update their research priorities. A NOSI describes specific topics of interest and will direct applicants to one or more active notice of funding opportunities for application submission. A NOSI is not a NOFO and is listed as a notice in the NIH Guide for Grants and Contracts. Applicants should read NOSIs carefully for any special requirements related to that specific announcement.
For more information about NOSIs sponsored by HDRP, Visit HDRP’s website and read more in our Funding Opportunities section of this newsletter.
COVID-19 Updates and Funding Opportunities
NIH is working to accelerate the development and delivery of therapeutic interventions, vaccines, and diagnostics for COVID-19. For more information, visit the latest research information from NIH.
NCI is mobilizing its scientific experts and cutting-edge resources to conduct research on COVID-19. For information about ongoing research initiatives, visit NCI COVID-19 Research Initiatives.
NCI COVID-19 Funding Opportunities
Click the links below for information about these funding opportunities, eligibility, and the application and review process.
- NOT-OD-20-097 - Availability of Administrative Supplements and Urgent Competitive Revisions for Research on the 2019 Novel Coronavirus and the Behavioral and Social Sciences
- PA-20-172 - Long-Term Effects of Disasters on Health Care Systems Serving Health Disparity Populations (R01- Clinical Trial Optional)
For information about funding opportunities sponsored by other institutes and centers at NIH, visit the latest research information from NIH.
COVID-19 Information for NIH Applicants and Grant Recipients
NIH is deeply concerned for the health and safety of people involved in NIH research, and about the effects on the biomedical enterprise in the areas affected by the HHS declared public health emergency for COVID-19. Due to the potential exceptional impact, we want to assure our grant recipient community that NIH will be doing its part to help you continue your research.
View this video from NIH’s Office of Extramural Research Director, Mike Lauer, regarding information for applicants and recipients of NIH Funds on Flexibilities Needed for COVID-19 Public Health Emergency.
from NIH’s Office of Extramural Research Director, Mike Lauer, regarding information for applicants and recipients of NIH Funds on Flexibilities Needed for COVID-19 Public Health Emergency.
For up-to-date information, guidance, and resources, visit the latest research information from NIH .
.
COVID-19 is an emerging, rapidly evolving situation and NIH/NCI is committed to keeping you informed.
- What people with cancer should know: Coronavirus: What People with Cancer Should Know

- The latest public health information from CDC: Coronavirus Disease 2019 (COVID-19)

HDRP Funding Announcements
Despite the challenges posed by the COVID-19 pandemic, NCI remains committed to funding research that will improve the health and quality of life of people diagnosed with cancer.
This section includes links to funding opportunities for healthcare delivery research.
Notices of Special Interest (NOSI)
NOSI: NCI’s Interest in Research to Improve Interprofessional Teamwork and Coordination During Cancer Diagnosis and Treatment, NOT-CA-19-059
Contact: Sallie Weaver
Expiration Date: January 8, 2022
View the archived webinar.
NOSI: Research to Improve the Interpretation of Patient-Reported Outcomes at the Individual Patient Level for Use in Clinical Practice, NOT-OD-20-079
Contact: Ashley Wilder Smith
Expiration Date: January 8, 2022
NOSI: De-implementation of Ineffective or Low-value Clinical Practices along the Cancer Care Continuum, NOT-CA-20-021
Contact: Erica Breslau
Expiration Date: May 10, 2022
View the archived webinar.
NOSI: Health Services Research on Minority Health and Health Disparities, NOT-MD-20-011
Contact: Brenda Adjei
Expiration Date: September 8, 2022
NOSI: Tailoring Follow-up Care for Survivors Using Risk-Stratified Pathways, NOT-CA-21-019
Contact: Michelle Mollica
Expiration Date: February 5, 2023
NOSI: Research on Oral Anticancer Agents in the Contexts of Utilization, Adherence, and Health Care Delivery, NOT-CA-20-026
Contact: Kate Castro
Expiration Date: May 8, 2023
Other Funding Opportunities
Using Information Technology to Support Systematic Screening and Treatment of Depression in Cancer, PA-18-493 (R01), PA-18-492 (R21)
Contact: Gurvaneet Randhawa
Expiration Date: May 8, 2021
Surgical Disparities Research, PAR-20-079 (R01)
Contact: Brenda Adjei
Expiration Date: July 8, 2021
Investigator-initiated Research on Genetic Counseling Processes and Practices (R21 Clinical Trial Optional), RFA-HG-20-048 (R01)
Contact: Erica Breslau
Expiration Date: July 8, 2021
Linking the Provider Recommendation to Adolescent HPV Vaccine Uptake, PAR-19-360 (R01), PAR-19-358 (R21), PAR-19-359 (R03)
Contact: Veronica Chollette
Expiration Date: September 8, 2022 (R01 and R21); July 17, 2022 (R03)
View the archived webinar.
End-of-Life and Palliative Needs of Adolescents and Young Adults (AYA) with Serious Illnesses, PAR-19-136 (R01), PAR-19-153 (R21)
Contact: Ashley Wilder Smith
Expiration Date: January 8, 2022
Increasing Uptake of Evidence-Based Screening in Diverse Adult Populations, PA-18-932 (R01)
Contact: Erica Breslau
Expiration Date: January 8, 2022
Dissemination and Implementation Research in Health, PAR-19-274 (R01), PAR-19-275 (R21), PAR-19-276 (R03)
Contact: Gila Neta, Wynne E. Norton, and David Chambers (DCCPS Implementation Science Team)
Expiration Date: May 8, 2022
Surgical Disparities Research, PAR-20-079 (R01)
Contact: Brenda Adjei
Expiration Date: July 6, 2022
Intervening with Cancer Caregivers to Improve Patient Health Outcomes and Optimize Health Care Utilization, PAR-19-352 (R01), PAR-19-355 (R21)
Contact: Michelle Mollica
Expiration Date: September 8, 2022
View the archived webinar.
Advancing Research to Develop Improved Measures and Methods for Understanding Multimorbidity (R01 Clinical Trial Optional), PAR-20-179 (R01)
Contact: Bryan Kim
Expiration Date: September 8, 2023
Identifying Innovative Mechanisms or Interventions that Target Multimorbidity and Its Consequences (R01 Clinical Trial Optional) PAR-20-180 (R01)
Contact: Bryan Kim
Expiration Date: September 8, 2023
For more information about funding including additional notice of funding opportunities, please visit our Funding page.
Research Highlights
2019-2020 NCI/AcademyHealth Visiting Scholars Program: Dr. Shellie Ellis, Putting Rural Research on the Map: Capacity for Cancer Care Delivery Research in Rural America
In 2017, the Healthcare Delivery Research Program launched a new NCI/AcademyHealth Visiting Scholar Program in partnership with AcademyHealth. Dr. Shellie Ellis, Associate Professor at the University of Kansas Department of Population Health, recently completed her tenure as the second NCI/AcademyHealth Visiting Scholar. Dr. Ellis spent the year working with Kate Castro and other members of the Healthcare Delivery Research Program to understand the capacity of the NCI Community Oncology Research Program (NCORP) to conduct cancer care delivery research in rural oncology practices.
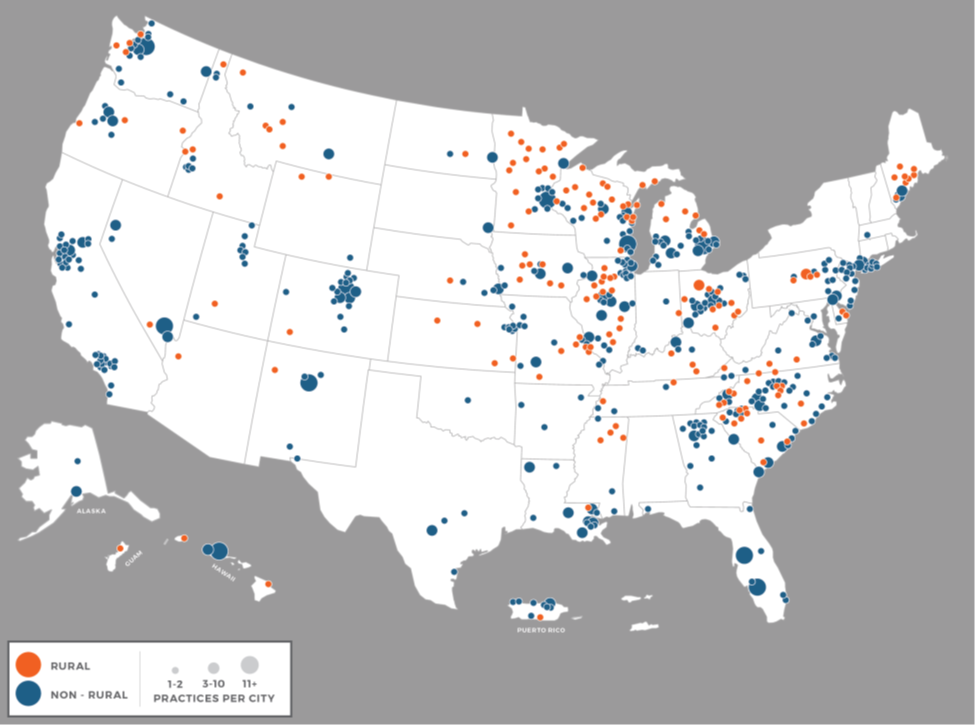
NCORP is a national network conducting cancer treatment, control, prevention, and care delivery studies in the communities where patients live. Dr. Ellis’ research indicated that 17% of practices participating in NCORP in the program’s first five years were located in areas classified by HRSA as rural, which is close to the proportion of rural population in the U.S. Two thirds of the NCORPs included at least one rural practice among their affiliates. Further, almost 10% of all cancer care delivery research (CCDR) accrual originates from rural practices. However, there is variability in the number of CCDR participants rural sites accrue, with half of the rural sites contributing to CCDR.
The study explored barriers and best practices for CCDR through a qualitative study of 22 rural NCORP practices. The qualitative results highlighted the heterogeneity of rural communities and cancer care delivery contexts across the NCORP network, ranging from affluent retirement communities to high concentrations of vulnerable populations, and from fully-staffed multidisciplinary cancer centers to care provided sporadically by oncologists serving multiple locations and facing high turnover among research staff. Practices described challenges with aligning participants, providers and the study protocols in many rural settings. Negative social determinants and transportation issues, coupled with challenges in research staffing and physician coverage, were found to limit patients’ ability to participate in research. Transportation incentives, centralized research training, and staffing structures which promote retention of research staff were thought to help. Lowering study start up burdens and aligning recruitment goals with the variable patient volumes across practices within the NCORP may further encourage more NCORPs to open CCDR studies at rural sites. Widening CCDR study eligibility criteria and recognizing and supporting the different organizational structures which exist in rural cancer programs may allow more rural sites to participate and benefit from the research conducting in NCORP. Participants suggested that studies aligned with rural practices’ priorities, engaging rural providers in care delivery interventions, and assessing outcomes on rural health systems may increase rural practice participation in cancer care delivery research.
Results from the studies are being prepared for publication.
As geographic disparities in cancer mortality continue to widen, the need to conduct robust research on urban-rural differences as well as rural-specific care delivery innovations will only escalate. The NCORP network has the capacity to do this work and, as more rural practices have been incorporated into the network in the most recent funding cycle, is poised to address this challenge.
If you would like to learn more about Shellie’s research, listen to her AcademyHealth webinar .
.
The 2021 Multilevel Intervention Training Institute (MLTI)
The inaugural Multilevel Intervention Training Institute (MLTI) was launched virtually in 2020. More than learning a new analytic approach, public health researchers require a foundational understanding of multilevel theory (models and frameworks), measurement (quantitative, qualitative and mixed methods), and analysis to become proficient in the design and conduct of multilevel research interventions within cancer care delivery.
The first part of the course focused on providing guidance on how to improve theory by integrating a multilevel perspective. The second part of the course included a series of lectures regarding issues related to multilevel measurement and research design, serving as the bedrock on which theory testing rests. The third part of the course dealt with testing hypotheses with multilevel analyses, including mediation, moderation, cross- and integrative-level models. The final part of the course included lectures on multilevel research, with scholars providing perspective on their experience, discussing challenges, and seminal work that influenced their thinking and work.
The 2020 program, which trained 50 MD/PhD individuals from diverse cancer backgrounds, was highly successful, with positive feedback from faculty and trainees. The 2021 Program will take place from February 18, 2021 through July 22, 2021. Click here for more information about the 2021 MLTI.
Future of Cancer Health Economics Research Virtual Conference
On December 2-3, 2020, HDRP hosted a virtual conference to identify challenges for conducting cancer health economics research and develop suggestions to support the development of this field. Over 400 individuals registered for the conference, including stakeholders from health care delivery organizations, academia, government, non-profit organizations, and pharmaceutical/biotechnology companies. The conference included discussions of NCI activities related to cancer health economics research; potential next steps to enhance this field across the cancer control continuum; and approaches to improve research focusing on communications, data resources, and health equity. Conference participants provided additional input during networking discussion sessions and breakout sessions. More information on the conference including archived recordings and presentation slides, will be available soon.
Upcoming Events
Telehealth Cyber Discussion Webinar Series
- June 11, 2021 - Enhancing Cancer Care of Rural Dwellers Through Telehealth and Engagement, Dr. Debra Friedman, Vanderbilt University Medical Center.
- TBD - Optimizing Telehealth Across the Cancer Care Continuum During the COVID 19 National Emergency, Dr. Ana Maria Lopez
 , Sidney Kimmel Cancer Center at Jefferson.
, Sidney Kimmel Cancer Center at Jefferson.
For other upcoming events please check our News & Events page. Events are updated regularly. To view our archived events, check our Events page.
Data and Resources
This section features information from a select number of HDRP’s data resources. A complete list of HDRP’s data and resources is available here.
National Health Interview Survey (NHIS)
The National Center for Health Statistics (NCHS) anticipates release of the 2019 NHIS data files and supporting documentation in fall 2020. The 2019 NHIS includes a 5-minute Cancer Control Supplement (CCS) that focused on colorectal, breast, cervical, and prostate cancer screening. The CCS was developed by NCI’s Division of Cancer Control and Population Sciences in collaboration with the Centers for Disease Control and Prevention’s (CDC) Division of Cancer Prevention and Control. Additionally, the 2020 CCS, currently being fielded, was adapted as of July 2020 to include specific questions about how COVID-19 has impacted individuals’ cancer treatment experiences. For more information, visit NHIS.
SEER-Medicare, SEER-Medicaid, SEER-CAHPS, SEER-MHOS
HDRP has developed a new video for the SEER linkages (SEER-MHOS, SEER-CAHPS, and SEER-Medicare), explaining the distinctions between these important data resources! Watch the video and for more information, visit SEER-Medicare, SEER-CAHPS, SEER-MHOS.
and for more information, visit SEER-Medicare, SEER-CAHPS, SEER-MHOS.
SEER-Medicare
We have completed the SEER-Medicare linkage update and have started taking applications for the new data, which includes cancer cases diagnosed from 1999-2017 and Medicare data from 1999-2018.
MEPS Experiences with Cancer Survivorship Supplement
The Medical Expenditure Panel Survey (MEPS) cancer working group has begun discussing a new Experience with Cancer supplemental questionnaire for a future MEPS. New questions on this supplement may include use of navigation services, participation in survivorship care programs, chronic/long-term symptoms, social isolation, and conversations with health care providers about clinical trial participation. For more information, visit MEPS.
