HDRP Newsletter, January 2020
Message from the HDRP Associate Director

New Year’s Greetings to Everyone,
On behalf of all my colleagues in the Healthcare Delivery Research Program (HDRP), I wish you a happy and healthy new year (and new decade). We look forward to continuing to work with you to advance our shared goal of advancing innovative research to improve the delivery of cancer-related care.
In this first newsletter of 2020, we highlight several funding opportunities, upcoming events, and data resources. We also introduce our new NCI/AcademyHealth Visiting Scholar, describe the work of our outgoing Visiting Scholar, and present some preliminary information about the portfolio of grants currently managed by HDRP.
I welcome you to reach out to me or any of our staff to learn more about the work of HDRP and the resources and services we provide. A full staff listing is available on our website.
Updates and Portfolio Analysis
NCI/AcademyHealth Healthcare Delivery Research Visiting Scholar 2020
In 2017, HDRP partnered with AcademyHealth to launch the NCI/AcademyHealth Healthcare Delivery Research Visiting Scholars Program. This program provides a year-long, funded, part-time opportunity to mid-career researchers to develop and pursue new research aims that advance both their own career goals and the field of healthcare delivery research more broadly.
to launch the NCI/AcademyHealth Healthcare Delivery Research Visiting Scholars Program. This program provides a year-long, funded, part-time opportunity to mid-career researchers to develop and pursue new research aims that advance both their own career goals and the field of healthcare delivery research more broadly.

Dr. Shellie Ellis has been selected to participate in the NCI/AcademyHealth Visiting Scholar Program. Her combined training in medical anthropology and health services research has allowed Dr. Ellis to be an early contributor to the field of implementation science. She has undertaken interventions to improve the use of evidence in screening and treatment across a variety of primary and specialty settings in profit and non-profit health organizations based in rural, urban, and suburban settings.
has been selected to participate in the NCI/AcademyHealth Visiting Scholar Program. Her combined training in medical anthropology and health services research has allowed Dr. Ellis to be an early contributor to the field of implementation science. She has undertaken interventions to improve the use of evidence in screening and treatment across a variety of primary and specialty settings in profit and non-profit health organizations based in rural, urban, and suburban settings.
During her time at NCI, Dr. Ellis will work with Ms. Kate Castro and Dr. Ann Geiger on projects related to understanding the barriers to and facilitators of cancer care delivery research in rural-serving oncology practices participating in the NCI Community Oncology Research Program (NCORP).
HDRP Grant Portfolio
In FY 2019, HDRP funded 52 new grants, totaling over $31 million dollars. These grants supported research across the cancer continuum to improve cancer prevention, detection, diagnosis, treatment and survivorship care.
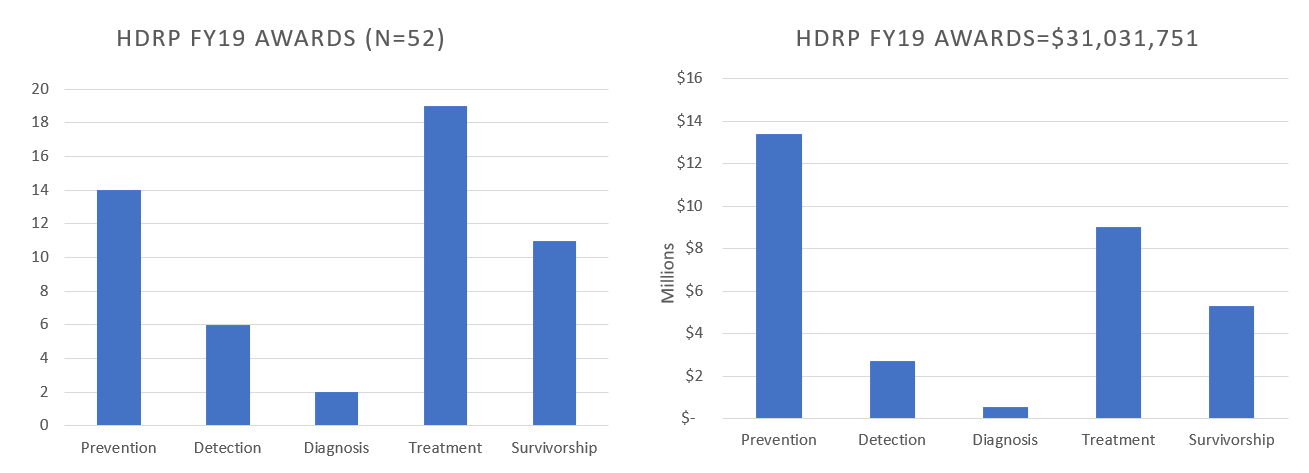
For a complete listing of all grants currently funded by the Healthcare Delivery Research Program, click here .
.
Research Highlights
Improving quality of cancer survivorship care: from framework to action
In 2017, the Healthcare Delivery Research Program launched a new NCI/AcademyHealth Visiting Scholar Program in partnership with AcademyHealth. Dr. Larissa Nekhlyudov, Brigham & Women’s Hospital/Dana-Farber Cancer Institute, was selected as the first scholar to participate in the program. During her time at NCI, she worked closely Dr. Michelle Mollica and other members of HDRP to develop a comprehensive, evidence-based cancer survivorship care quality framework (see figure below). In a recent AcademyHealth blog and article published in the Journal of the National Cancer Institute
and article published in the Journal of the National Cancer Institute , Dr. Nekhlyudov and colleagues described the framework and proposed the next steps to systematically apply it in clinical settings, research, and policy. In order to consider how the framework may be moved into implementation, HDRP organized a stakeholder meeting in December 2018, with representation from researchers, clinicians, patient advocates, insurers and policy makers.
, Dr. Nekhlyudov and colleagues described the framework and proposed the next steps to systematically apply it in clinical settings, research, and policy. In order to consider how the framework may be moved into implementation, HDRP organized a stakeholder meeting in December 2018, with representation from researchers, clinicians, patient advocates, insurers and policy makers.
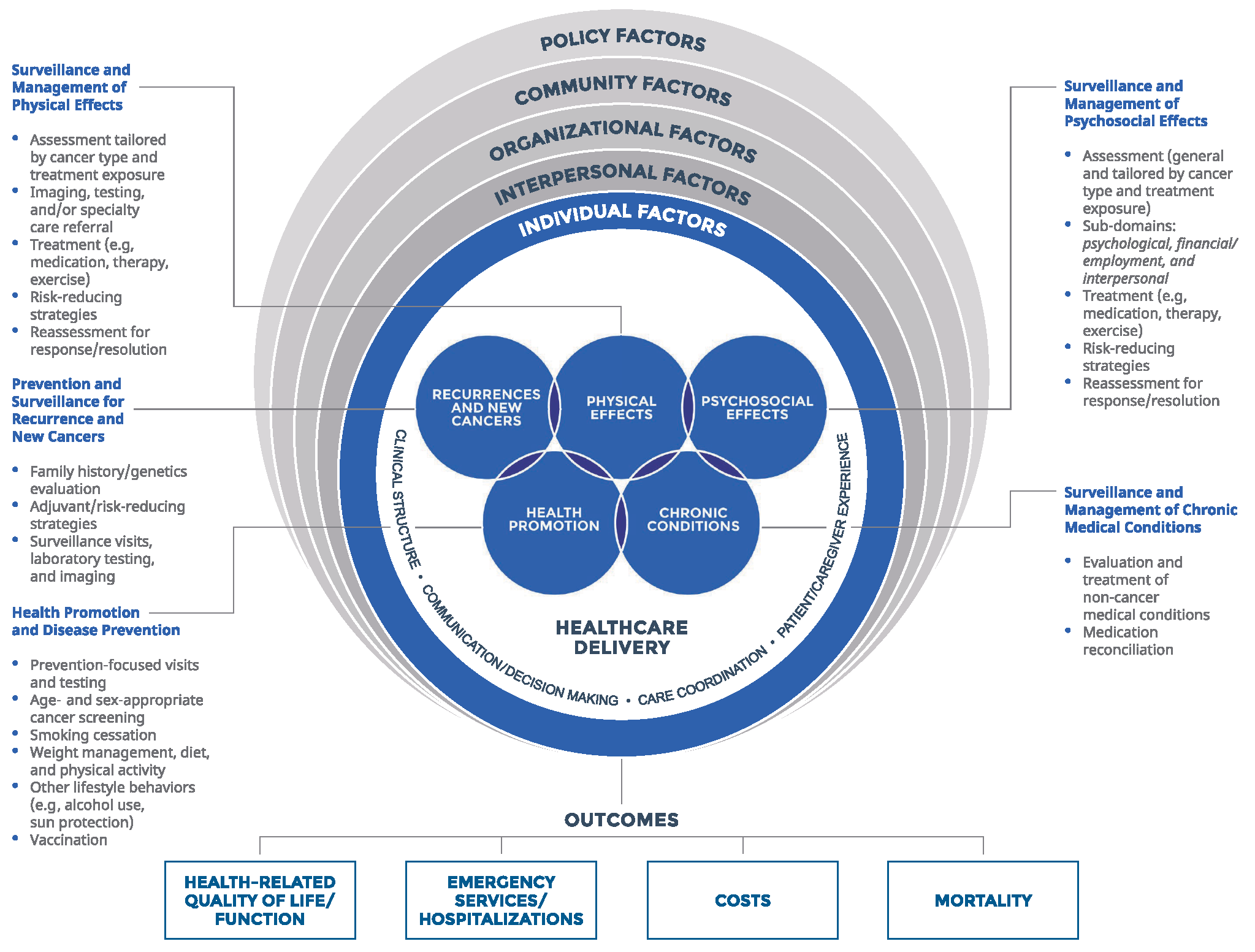
Key points from meeting:
- Selection and prioritization of quality domains: There is a need to measure the quality of cancer survivorship care in different settings and programs, and across different cancer types. This requires the selection and prioritization of quality measures, taking into account factors such as patient goals and needs, feasibility, impact, evidence base, as well as potential burden to the patient, provider and healthcare system.
- Implementation of the framework: The developed framework is complex and implementing it across all domains in clinical settings may be challenging. However, the entire framework may not need to be fully implemented in all situations. It may be possible to focus on selected domains and measures that may serve as benchmarks for quality assessment over time.
- Evaluation of the evidence: The framework can guide an evaluation of the state of the science of cancer survivorship care. By synthesizing existing research and identifying gaps, we can move into implementing evidence-based interventions, de-implementing low-value interventions, and generating additional needed evidence.
- Consideration of payment for delivering quality survivorship care: While survivorship care is an essential component of overall cancer care, there is still a need to develop a reimbursement strategy, including appropriate attribution and accountability, for the provision of quality survivorship care.
- Education of providers, patients and caregivers: This framework can serve as a tool to educate providers, patients, and caregivers regarding the essential components of cancer survivorship care.
Since the stakeholder meeting, HDRP and NCI’s Office of Cancer Survivorship (OCS) have been engaged in several efforts to enhance the quality of cancer survivorship care. First, OCS recently released revised definitions of the terms cancer survivor and cancer survivorship research . These definitions include an adapted version of the quality framework as it applies to cancer survivorship research. In addition, HDRP recently released an RFA in 2019, entitled, Optimizing the Management and Outcomes for Cancer Survivors Transitioning to Follow-Up Care (RFA-CA-19-035) with awards expected in 2020. The goal of this RFA is to support grants that develop and test innovative models of care for adult survivors of cancer who are transitioning from active treatment to follow-up care.
. These definitions include an adapted version of the quality framework as it applies to cancer survivorship research. In addition, HDRP recently released an RFA in 2019, entitled, Optimizing the Management and Outcomes for Cancer Survivors Transitioning to Follow-Up Care (RFA-CA-19-035) with awards expected in 2020. The goal of this RFA is to support grants that develop and test innovative models of care for adult survivors of cancer who are transitioning from active treatment to follow-up care.
As the population of cancer survivors in the U.S. continues to grow to 26 million over the next few decades, the need to provide comprehensive cancer survivorship care will only escalate. Care for cancer survivors is complex, and this framework can serve as a tool to guide future measurement and improvements in care quality.
Organizational Research in Healthcare Workshop
On October 15, 2019, HDRP held a one-day workshop to review the state-of-science on the study of healthcare organizations as applied to cancer care delivery. This workshop brought together scientific leaders in health services research, practicing clinicians, and researchers and provided insight into future research directions that support HDRP’s mission to advance the evidence-based practice of healthcare delivery. For more information about the workshop and to view archived materials, see the archived workshop page.
Funding Opportunities
Notice of Special Interest (NOSI):
NOSI: NCI’s Interest in Research to Improve Interprofessional Teamwork and Coordination During Cancer Diagnosis and Treatment, NOT-CA-19-059
Contact: Sallie Weaver
Expiration Date: January 8, 2022
Click here to view the archived webinar.NOSI: De-implementation of Ineffective or Low-value Clinical Practices along the Cancer Care Continuum, NOT-CA-20-021
Contact: Erica Breslau
Expiration Date: May 10, 2022
Other Funding Opportunities:
Multilevel Interventions in Cancer Care Delivery: Follow-up to Abnormal Screening Tests, PA-17-495 (R01)
Contact: Erica Breslau
Expiration Date: January 8, 2021Using Information Technology to Support Systematic Screening and Treatment of Depression in Cancer PA-18-493 (R01) and PA-18-492 (R21)
Contact: Gurvaneet Randhawa
Expiration Date: May 8, 2021Linking the Provider Recommendation to Adolescent HPV Vaccine Uptake; PAR-19-360 (R01), PAR-19-358 (R21), PAR-19-359 (R03)
Contact: Sarah Kobrin
Expiration Date: September 8, 2022 (R01 and R21); July 17, 2022 (R03)
Click here to view the archived webinar.End-of-Life and Palliative Needs of Adolescents and Young Adults (AYA) with Serious Illnesses, PAR-19-136 (R01), PAR-19-153 (R21)
Contact: Ashley Wilder Smith
Expiration Date: January 8, 2022Increasing Uptake of Evidence-Based Screening in Diverse Adult Populations, PA-18-932 (R01)
Contact: Erica Breslau
Expiration Date: January 8, 2022Dissemination and Implementation Research in Health, PAR-19-274 (R01), PAR-19-275 (R21), PAR-19-276 (R03)
Contact: Gila Neta, Wynne E. Norton, and David Chambers (DCCPS Implementation Science Team)
Expiration Date: May 8, 2022Surgical Disparities Research, PAR-20-079 (R01)
Contact: Brenda Adjei
Expiration Date: July 6, 2022Intervening with Cancer Caregivers to Improve Patient Health Outcomes and Optimize Health Care Utilization; PAR-19-352 (R01), PAR-19-355 (R21)
Contact: Michelle Mollica
Expiration Date: September 8, 2022
Click here to view the archived webinar.
For more information about funding including additional notice of funding opportunities, please visit our funding page.
Grants Announcements
NCI Budget and Funding Policy for FY2020
Information on the NCI’s FY 2020 budget can be found on the NCI Office of Budget and Finance website . Information about NCI’s funding strategy and paylines are available from the Division of Extramural Activities. Please click here
. Information about NCI’s funding strategy and paylines are available from the Division of Extramural Activities. Please click here for additional information.
for additional information.
Notice of Funding Opportunities (NOFOs) for Research Answers to NCI’s Provocative Questions
The National Cancer Institute (NCI) has published Notice of Funding Opportunities (NOFOs) as Request for Applications (RFAs) to solicit applications responding to the new set of Provocative Questions (PQ). The NOFOs will utilize the R01 and R21 activity codes.
The PQ Initiative includes a new set of 9 PQs, 2 of which are directly relevant to HDRP:
- PQ7: What methods can be developed to integrate patient-generated health data into electronic health records?

- PQ8: What strategies improve and sustain the coordination of comprehensive healthcare for underserved cancer patients with comorbid conditions?

Additional information defining the research scope for responsive projects is provided in the NOFOs and posted at the Provocative Questions website .
.
NIH Expanding Usage of Notices of Special Interest (NOT-OD-19-107)
This notice informs the extramural community that NIH is expanding and formalizing the use of Notices of Special Interest (NOSI) posted in the NIH Guide for Grants and Contracts to announce interest in specific scientific research topics.
How Does a NOSI Work?
A NOSI is a standard, formal format for NIH institutes to share and update their research priorities. A NOSI describes specific topics of interest and will direct applicants to one or more active notice of funding opportunities for application submission. A NOSI is not a NOFO and is listed as a notice in the NIH Guide for Grants and Contracts . Applicants should read NOSIs carefully for any special requirements related to that specific announcement.
. Applicants should read NOSIs carefully for any special requirements related to that specific announcement.
HDRP currently sponsors two NOSIs. For more information, visit HDRP’s website and read more in our Funding Opportunities section of this Newsletter.
Upcoming Events
Application deadline for the Multilevel Intervention Training Institute – January 20, 2020
Webinar: Enhancing survivorship care for survivors with metastatic cancer – January 22, 2020
Webinar: Healthcare Teams Cyber Discussion Series: Expansion of an Oncology Care Coordination Nurse Model to Reduce Hospitalizations and Emergency Department Utilization – February 25, 2020
Webinar: Healthcare Teams Cyber Discussion Series: The Evolving Role of the Financial Counselor in an Oncology Care Model Pilot Program – May 26, 2020
For other upcoming events please check our News & Events page. Events are updated regularly.
Data and Resources
Release of 2018 National Health Interview Survey (NHIS) Data:
The National Center for Health Statistics (NCHS) released the 2018 NHIS data files and supporting documentation in June. The 2018 NHIS includes a 5-minute Cancer Control Supplement (CCS) that focused on cancer screening. The CCS was developed by NCI’s Division of Cancer Control and Population Sciences in collaboration with the Centers for Disease Control and Prevention’s (CDC) Division of Cancer Prevention and Control. The 2018 NHIS data can be downloaded from the NCHS website
 .
.SEER-Medicare:
During 2020, NIH will be updating the SEER-Medicare data to include SEER cases diagnosed through 2017 and their Medicare enrollment and claims data through 2018. The updated data will be made available to the research community by the end of 2020. NCI staff are writing a collection of manuscripts, which will be published in 2020 as a JNCI supplement. These manuscripts will demonstrate ways to optimize data previously included in the SEER-Medicare linkage, explore the addition of new data included in the SEER-Medicare linkage, and evaluate new data linkages, including SEER-Medicaid.
SEER-Medicaid:
NIH is finalizing a linkage of SEER cases diagnosed between 2006-2013 with Medicaid enrollment data (2006-2013) obtained from the Centers for Medicare & Medicaid Services for all 50 states plus the District of Columbia. The linked data will be released in early 2020.
HealthMeasures:
Over the last 15 years, the NIH invested in the development and extensive testing of four state-of-the-science validated patient-centered outcomes measurement systems designed to capture information about symptoms and functioning (e.g., pain, fatigue, and physical function) directly from patients. These tools include PROMIS
 , The NIH Toolbox
, The NIH Toolbox , Neuro-QoL
, Neuro-QoL , and ASCQ-Me
, and ASCQ-Me .
.In 2014, each of these four systems, developed under separate NIH funding sources, were brought together and made available to the public as a uniform resource. Known collectively as HealthMeasures
 , the resource was supported by a trans-NIH cooperative agreement (U2CCA186878) supported by 14 NIH Institutes and Centers.
, the resource was supported by a trans-NIH cooperative agreement (U2CCA186878) supported by 14 NIH Institutes and Centers.This year marks the historic conclusion of the NIH grant supporting HealthMeaures with full expectations that HealthMeasures will continue to remain available to the research community at little or no cost, and that NIH grantees will continue using these tools in advancing NIH-supported research.
SEER-CAHPS:
SEER-CAHPS is a data resource based on a linkage between the NCI’s Surveillance, Epidemiology and End Results (SEER) cancer registry data and the Centers for Medicare & Medicaid Services’ (CMS) Medicare Consumer Assessment of Healthcare Providers and Systems (CAHPS®) surveys. During 2020, NIH will be updating the SEER-CAHPS data to include SEER cases diagnosed through 2017, Medicare CAHPS surveys through 2019, and Medicare enrollment and claims data (Fee-for-Service beneficiaries only) through 2018. The updated data will be made available to the research community by the end of 2020. We have also recently updated our guidance and support for researchers, including a 2019 webinar focused on methodological considerations of the resource.
SEER-MHOS:
In Spring 2019, NIH updated the SEER-MHOS publicly available data to include MHOS cohort data through 2017 and SEER cases diagnosed through 2015. Medicare Part D Prescription Drug data is now available in this resource for Medicare Advantage enrollees with prescription drug coverage. Two new informational webinars are now available at the SEER-MHOS News & Information page.
MEPS Experiences with Cancer Survivorship Supplement:
The Medical Expenditure Panel Survey (MEPS), a nationally representative survey of the civilian noninstitutionalized population in the United States, collects comprehensive data on health insurance, access to health care, utilization, and expenditures. In 2011 and 2016, the NCI in collaboration with the American Cancer Society (ACS), the Centers for Disease Control and Prevention (CDC) and LIVESTRONG funded the Experiences with Cancer Supplement to provide additional information about cancer survivors’ financial burden, access to medical care, and employment patterns for themselves and their caregivers. The Experiences with Cancer Supplements and other MEPS data are available from the Agency for Healthcare Research and Quality (AHRQ).
Patterns of Care:
Training for the 2019 Patterns of Care study, focused on advanced non-small cell lung cancer (NSCLC) and melanoma, was held at NCI on October 24, 2019. Abstractors from all 12 participating registries attending the training and provided important updates for the planned data collection. This study will collect detailed information on tumor mutations, targeted therapies, symptom assessment/management, and financial conversations between patients and health care providers.
